 |
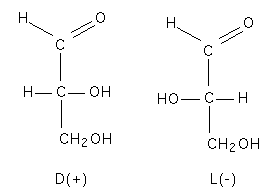
| Asymmetry in glyceraldehyde |
Sugars are molecules that have a generic formula of CnH2nOn. They can be descibed as polyhydroxy compounds containing either a ketose of aldehyde group. Most sugars are optically active and this comes from the presence of asymmetrically substituted carbons in the sugar. Glyceraldehyde is the simplest sugar molecule with optical activity. D-glyceraldehyde shifts light to the right (+) whereas L-glyceraldeyde shifts light to the left.
Different sugar conformations
These flat linear drawings are known as Fisher projections and the relative position of the first asymmetric carbon on sugar chain (relative to the non reducing end) determines if the sugar is a D or L sugar. The (+) or (-) classification is the optical activity and is not directly related to the D and L conformations.
The numbering of carbon atoms in sugar systems is based on the Fisher project starting at the top as C1. Hence in a glucopyranose structure the numbering runs clockwise starts just below the ring oxygen with the final C6 carbon froming the -CH2OH side chain.
 |
| alpha sugar beta sugar |
However sugars can rotate through a wide range of structures and the reducing end can cyclise via one of the hydroxy groups to form ring structures, usually five or six membered rings. These ring structures can flip between different conformations themselves and the most common forms are known as the chair conformations. The two different chair conformations get their names from the relative positions of the 1 and 4 carbon positions to the main sugar plane as defined by the O-C2-C3-C5 plane. There is also a conformation known as the boat but in most sugars this is not very favoured energetically.
 |
